The artificially created element 117, tennessine (ts), may also be a halogen. 1).although this interaction is a relative newcomer to the supramolecular chemistry arena, it has a long history.
List Of What Is Halogen In Chemistry For Small Room, Haloalkanes, commonly called alkyl halides, are a class of chemical. The halogens, aka halogen family, are a group of reactive elements in group 17 of the periodic table, to the right of the chalcogens, and to the left of the noble gases.
 The properties and reactions of the halogens YouTube From youtube.com
The properties and reactions of the halogens YouTube From youtube.com
The halogens are a family of chemical elements that comprise an entire column of the periodic table. (chemistry) forming names of chemical compounds which contain one or more halogen atoms. What is halo in chemistry? Halogens are elements that are located in group 17 of the periodic table.
The properties and reactions of the halogens YouTube The halogen elements are fluorine, chlorine, bromine, iodine, astatine, and possibly tennessine.
The halogens, aka halogen family, are a group of reactive elements in group 17 of the periodic table, to the right of the chalcogens, and to the left of the noble gases. Fluorine and chlorine are the “poster children” of the halogens. Fluorine (f), chlorine (cl), bromine (br), iodine (i), and astatine (at). A halogen has 7 valence electrons.
 Source: aim2destiny.blogspot.com
Source: aim2destiny.blogspot.com
Any of the five elements fluorine, chlorine, bromine, iodine, and astatine that form part of group viia of the periodic table and exist in the free state normally as diatomic molecules. In fact, halogens are so reactive that they do not occur as free elements in nature. A halogen is any element on the periodic table of elements that falls into group (or family) 17. KNOWLEDGE TO GAIN.
 Source: youtube.com
Source: youtube.com
Fluorine, chlorine, bromine, iodine (i), and astatine. In fact, halogens are so reactive that they do not occur as free elements in nature. In this regard, what is a halogen in chemistry? Chemistry TutorialHalogen Definition, Chemical Properties.

Halogen definition, any of the electronegative elements, fluorine, chlorine, iodine, bromine, and astatine, that form binary salts by direct union with metals. A halogen has 7 valence electrons. Halogens the halogens are a group of elements in the periodic table. Group 7 Halogens Presentation Chemistry.

The halogens are a group of elements in the periodic table. Fluorine, chlorine, bromine, iodine (i), and astatine. Fluorine and chlorine are the “poster children” of the halogens. 68 PERIODIC TABLE NOBLE GASES HALOGENS, TABLE HALOGENS.
 Source: youtube.com
Source: youtube.com
Many, however, are common in combination with other elements here is a look at the identity of these elements, their location on the periodic table, and their common. In fact, halogens are so reactive that they do not occur as free elements in nature. The most commonly found halogens in organic compounds are fluorine, chlorine, bromine, and iodine. The properties and reactions of the halogens YouTube.
 Source: pinterest.com
Source: pinterest.com
The halogens are the only periodic table group containing elements in all three familiar states of matter (solid, liquid, and gas) at standard temperature and pressure. Halogens are elements that are located in group 17 of the periodic table. Fluorine, chlorine, bromine, iodine (i), and astatine. Addition of Halogens to Alkenes with Dihalides Organic.
![Chemistry Halogen [PPTX Powerpoint] Chemistry Halogen [PPTX Powerpoint]](https://i2.wp.com/static.fdocuments.in/img/1200x630/reader020/image/20191006/546da58ab4af9fe51b8b4f2e.png?t=1601758656) Source: fdocuments.in
Source: fdocuments.in
Any of the five elements fluorine, chlorine, bromine, iodine, and astatine that form part of group viia of the periodic table and exist in the free state normally as diatomic molecules. Elements in the halogen group have seven electrons in their outer shells giving them many unique properties. The halogens are a group in the periodic table consisting of five chemically related elements: Chemistry Halogen [PPTX Powerpoint].
 Source: thoughtco.com
Source: thoughtco.com
They are reactive nonmetallic elements that form strongly acidic compounds with hydrogen, from which simple salts can be made. The halogens are a family of chemical elements that comprise an entire column of the periodic table. The halogen elements are fluorine, chlorine, bromine, iodine, astatine, and possibly tennessine. Halogen Elements and Properties.
 Source: sciencenotes.org
Source: sciencenotes.org
Halogens are elements that are located in group 17 of the periodic table. The halogens are a group in the periodic table consisting of five chemically related elements: Definition of ~ s 1) group viia elements: Halogen Elements List and Facts.
 Source: alamy.com
Source: alamy.com
Halogen bulbs contain a small amount of halogen gas. The term ‘halogen’ means �salt former�. How do you name halo alkanes? Halogens. Chemical elements of Periodic table. Vector.
 Source: mrseldred.weebly.com
Source: mrseldred.weebly.com
The halogens exist, at room temperature, in all three states of matter: Elements in the halogen group have seven electrons in their outer shells giving them many unique properties. Fluorine (f), chlorine (cl), bromine (br), iodine (i), and astatine (at). 6 Periodic Table Mrs. Eldred�s Classroom.

These elements appear in the column just to the left of the noble gases mentioned earlier. Chlorine, bromine and iodine are the three common group 7 elements. These elements are called the halogens (from the greek hals, salt, and gennan, to form or generate) because they are literally the salt formers. Chemistry Halogen.
 Source: britannica.com
Source: britannica.com
Halogens are elements that are located in group 17 of the periodic table. Physical states of halogens halogens represents all of the three familiar states of matter: Halogen chemistry, volume 3 focuses on advancement in the study of halogens. halogen Facts, Definition, Properties, & Uses Britannica.
 Source: chemizi.blogspot.com
Source: chemizi.blogspot.com
1).although this interaction is a relative newcomer to the supramolecular chemistry arena, it has a long history. Halogen bulbs contain a small amount of halogen gas. (entry 1 of 2) : Halogen elementsdefinitionpropertiesreactivity and uses.
 Source: youtube.com
Source: youtube.com
These elements appear in the column just to the left of the noble gases mentioned earlier. The halogens exist, at room temperature, in all three states of matter: How do you name halo alkanes? Group 7, The Halogens YouTube.
 Source: mydigitalkemistry.com
Source: mydigitalkemistry.com
In this regard, what is a halogen in chemistry? The halogens are the elements in group 17 of the periodic table. (entry 1 of 2) : Group VII A Elements Properties Halogens Chemistry.
 Source: chem.ucla.edu
Source: chem.ucla.edu
Organic halogen compounds are organic compounds that contain a single or more atom of hydrogen altered by an equivalent number of halogen atoms (f, cl, br, or i). In this regard, what is a halogen in chemistry? Fluorine and chlorine are the “poster children” of the halogens. Illustrated Glossary of Organic Chemistry Halogen.
 Source: physciq.blogspot.com
Source: physciq.blogspot.com
Fluorine (f), chlorine (cl), bromine (br), iodine (i), and astatine (at). The halogens are a family of chemical elements that comprise an entire column of the periodic table. Halogen atoms can be found in almost any type of organic substance (e.g., alcohols, ketones, and carboxylic acids). Physical And Chemical Properties Halogens PHYSCIQ.
 Source: byjus.com
Source: byjus.com
The halogens exist, at room temperature, in all three states of matter: The most commonly found halogens in organic compounds are fluorine, chlorine, bromine, and iodine. The most commonly found halogens in organic compounds are fluorine, chlorine, bromine, and iodine. Electronic Configuration Halogen Characterisitcs.
 Source: youtube.com
Source: youtube.com
Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. In the modern iupac nomenclature, this group is known as group 17. Halogens the halogens are a group of elements in the periodic table. Halogens YouTube.
 Source: spmchemistry.blog.onlinetuition.com.my
Source: spmchemistry.blog.onlinetuition.com.my
They are located to the right of the other nonmetals and to the left of the noble gases. The halogens are a group of elements in the periodic table. Fluorine (f), chlorine (cl), bromine (br), iodine (i), and astatine (at). Group 17 Elements Halogens SPM Chemistry.
 Source: thoughtco.com
Source: thoughtco.com
(left to right) chlorine is a gas, bromine is a liquid, and iodine is a solid. The artificially created element 117 may also be a halogen. The halogens are the elements in group 17 of the periodic table. List of Halogens (Element Groups).
 Source: thoughtco.com
Source: thoughtco.com
The halogens exist, at room temperature, in all three states of matter: The halogens are the only periodic table group containing elements in all three familiar states of matter (solid, liquid, and gas) at standard temperature and pressure. The halogens, aka halogen family, are a group of reactive elements in group 17 of the periodic table, to the right of the chalcogens, and to the left of the noble gases. List of Halogens (Element Groups).
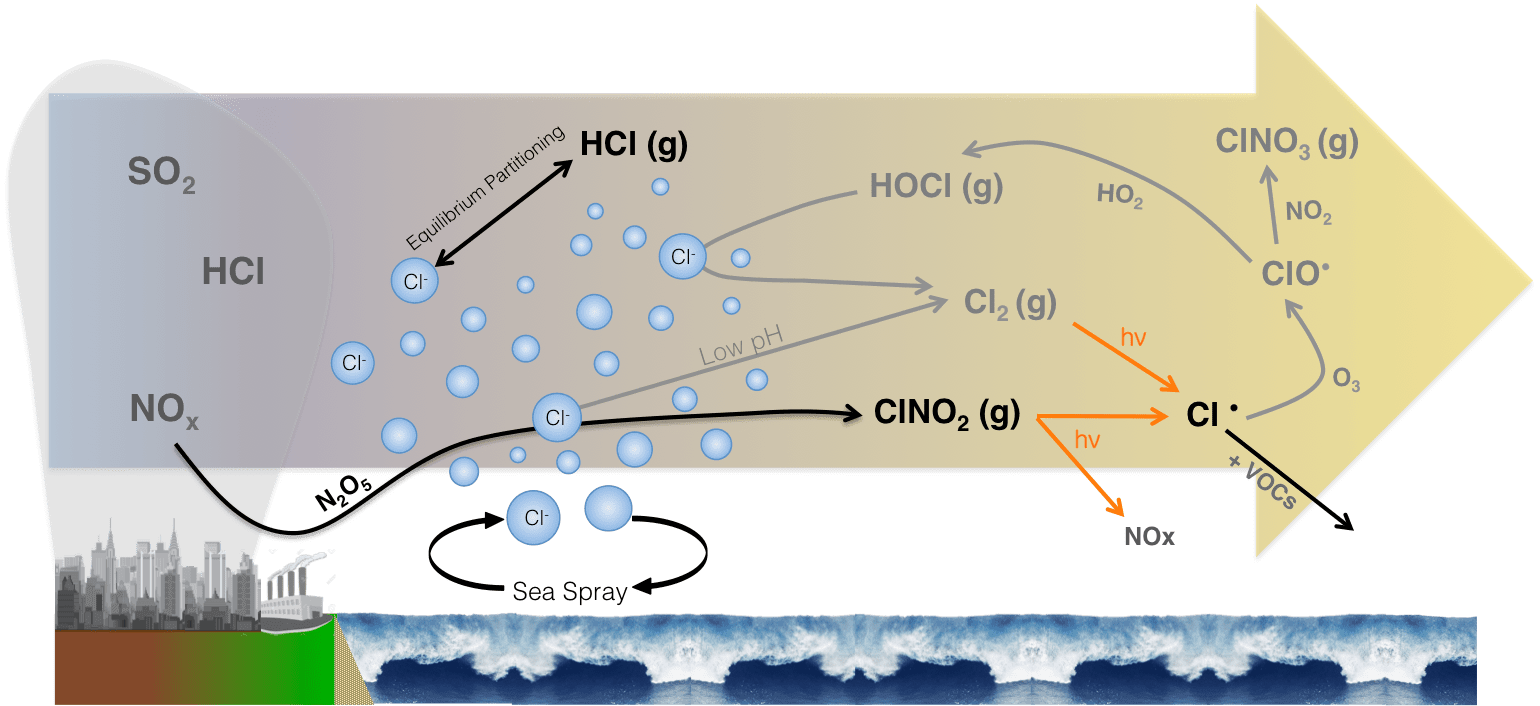 Source: atmos.washington.edu
Source: atmos.washington.edu
They are reactive nonmetallic elements that form strongly acidic compounds with hydrogen, from which simple salts can be made. In this regard, what is a halogen in chemistry? What is the halogen group? Halogen Chemistry The Thornton Lab.
 Source: byjus.com
Source: byjus.com
Organic halogen compounds are organic compounds that contain a single or more atom of hydrogen altered by an equivalent number of halogen atoms (f, cl, br, or i). What is halo in chemistry? Fluorine, chlorine, bromine, iodine (i), and astatine. Halogens Trends in Chemical and Physical Properties.
A Halogen Has 7 Valence Electrons.
1).although this interaction is a relative newcomer to the supramolecular chemistry arena, it has a long history. The halogens are the only periodic table group containing elements in all three familiar states of matter (solid, liquid, and gas) at standard temperature and pressure. The most commonly found halogens in organic compounds are fluorine, chlorine, bromine, and iodine. A halogen is any element on the periodic table of elements that falls into group (or family) 17.
Chlorine, Bromine And Iodine Are The Three Common Group 7 Elements.
Halogens are found in the environment only in the form of ions or compounds, because of. Fluorine, chlorine, bromine, iodine (i), and astatine. The term ‘halogen’ means �salt former�. The halogens, aka halogen family, are a group of reactive elements in group 17 of the periodic table, to the right of the chalcogens, and to the left of the noble gases.
The Halogens Exist, At Room Temperature, In All Three States Of Matter:
For more information, see the related links, below. Halogen definition, any of the electronegative elements, fluorine, chlorine, iodine, bromine, and astatine, that form binary salts by direct union with metals. The halogens are highly reactive nonmetallic elements. In this regard, what is a halogen in chemistry?
Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), And Astatine (At).
The artificially created element 117 may also be a halogen. In particular, studies of the interactions of molecular. The halogens are a family of chemical elements that comprise an entire column of the periodic table. What is the halogen group?







